
View of the structure of DNA by Виталий Смолыгин. License:Public Domain
DNA must be one of the most instantly recognisable molecules and many people will be familiar with its elegant double helical structure. I am a biochemist, and I have been privileged to work with this molecule for over thirty years, exploring its structure and function and how things interact with it. It is the most fascinating molecule in the universe!
Although its structure is elegant (some might even say beautiful), DNA is essentially a repetitive polymer composed of only four units (for which we use the abbreviations A, G, C and T). Yet despite its simplicity the order of these units, DNA defines many of our physical characteristics. Relatively small changes in the sequence account for differences between individuals and are responsible for inherited genetic conditions.
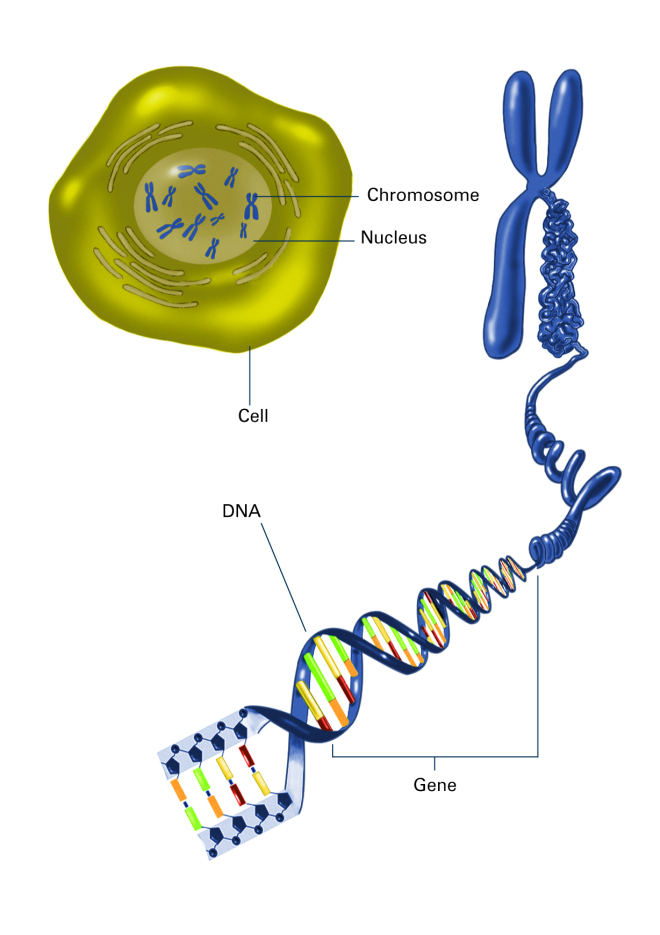
Chromosome inside nucleus by National Institute of General Medical Sciences. Available:http://1.usa.gov/1NhVQwm
The dimensions of this molecule are mind-blowing. The DNA strands in each cell in our body contain about 6 billioni repeating units, in a very precise order, which are arranged into 46 chromosomes and have a total length of about 2 metres. It’s incredibly thin (about 2 nanometresii in diameter), yet about 2 metres long (about a billioniii times longer than it is wide). If the DNA from one of your cells was scaled up to the thickness of string, it would be about 1000 kilometres long.
The DNA molecule is very stable – it has to be to survive in a watery environment, yet it is not rigid. Although we visualise short sections of DNA in the familiar helical structure, it is highly dynamic and flexible – it has to be in order to squeeze into the cell nucleus that may only be about 10 micrometersiv in diameter. Exactly how DNA is packaged so that the right parts are accessible in the right cells at the right times, is an area of ongoing research.
DNA must have emerged as the genetic material very early in the origin of life, as it (or its very close cousin RNA) is used by all living organisms from viruses to bacteria to fruit flies to humans. Is it chemically unique, or could any other molecule have done the job? When Steven Benner, a biological chemist, first set out to re-engineer DNA he commented, “the first thing you realize is that it is a stupid design.”v Would you really chose a molecule that had negatively charged parts on the outside, and which is held together by weak bonds that are easily disrupted by water? Yet the truth is much more fascinating.
![DNA Split By US Department of Energy (DOE Human Genome project) [Public domain], via Wikimedia Commons](https://www.faraday.cam.ac.uk/wp-content/uploads/2020/02/dna-split.png)
DNA Split By US Department of Energy (DOE Human Genome project) [Public domain], via Wikimedia Commons
Some of my research has been looking at other structures that DNA can adopt. Isolated DNA can form three and even four-stranded structures. In biochemistry, we know that if things are possible in the lab then natural processes have usually found a way to exploit them for biological benefit. Some of these ‘unusual’ structures may play important roles in controlling which genes are activated. It appears that hidden within the DNA sequences are subtle messages that are much more complex than a simple ‘code’.
The molecular design rules for constructing a DNA helix are very simple. A always pairs with T, and G pairs with C. These rules can be used to generate tiny DNA-based structures (nanostructures), even smiley faces, like molecular lego. It remains to be seen whether these will have useful applications, maybe for drug delivery or as molecular machines. For the moment they are simply fascinating and fun to work with; that’s one of the pleasures of being a scientist. The way the world operates is fascinating even if today’s discoveries don’t have any immediate application – you just don’t know what is next around the corner to be discovered.
The Psalmist looked up to the heavens and declared God’s glory. I wonder what he would have said about the way things appear at the molecular scale. For those of us who are privileged to study these molecules, they inspire a sense of wonder and awe. We are indeed fearfully and wonderfully made – right down to the very smallest components. This is not to invoke any special ‘design’ – this amazing molecule appeared early in the emergence of life and has been changing over billions of years. The mutations that drive evolution may be random, but they are simple chemical events and there is nothing ‘magical’ about them. But under God’s providential guidance and according to his natural laws, these apparently random events on a repeating polymer have generated the wonderful complexity of life.
 Keith Fox is Professor of Biochemistry at the University of Southampton, Associate Director of The Faraday Institute, and a former chair of Christians in Science. He originally studied in Cambridge before moving to Southampton as a lecturer in 1987. He is the senior executive editor of Nucleic Acids Research and editor of Science and Christian Belief. His research concerns the sequence specific recognition of DNA by small molecules. Several DNA-binding antibiotics are currently used in cancer chemotherapy, and we are seeking to understand the molecular mechanisms by which they bind to DNA with a view to designing new agents with improved selectivity.
Keith Fox is Professor of Biochemistry at the University of Southampton, Associate Director of The Faraday Institute, and a former chair of Christians in Science. He originally studied in Cambridge before moving to Southampton as a lecturer in 1987. He is the senior executive editor of Nucleic Acids Research and editor of Science and Christian Belief. His research concerns the sequence specific recognition of DNA by small molecules. Several DNA-binding antibiotics are currently used in cancer chemotherapy, and we are seeking to understand the molecular mechanisms by which they bind to DNA with a view to designing new agents with improved selectivity.
i. That is, US billions: 6,000,000,000. A British billion is 1,000,000,000,000. This is possibly the only case where something is bigger in the UK than the US!
ii. 1mm = 1,000,000 nanometres.
iii. Again, a US billion.
iv. 1mm = 1,000 micrometres.
v. Quoted in http://www.nature.com/news/chemical-biology-dna-s-new-alphabet-1.11863




